
January 19, 2026
 John Oliva/USA TODAY NETWORK via Imagn Images
John Oliva/USA TODAY NETWORK via Imagn Images
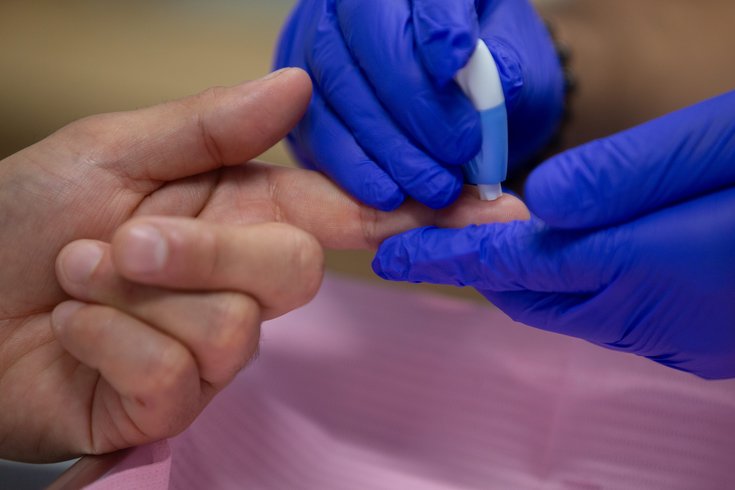
A new study of adults 60 and over is examining whether blood collected with a finger-prick can show proteins in the brain that are connected to Alzheimer's disease.
A new finger-prick blood test could help diagnose older adults with Alzheimer's disease.
Researchers are examining whether samples collected show accurately certain biomarkers which denote abnormal proteins in the brain. Volunteers in the ongoing study undergo cognitive tests, an MRI and PET scans to detect amyloid and tau proteins, which have been linked to Alzheimer's, and those results are compared with the blood samples to confirm a diagnosis.
The trial, called the Bio-Hermes-002 research study, is being conducted by the Global Alzheimer's Platform Foundation, LifeArc and the UK Dementia Research Institute. Approximately 1,000 adults over the age of 60 in the U.S., United Kingdom and Canada will be tested. So far, 883 of the 1,000 participants have been enrolled, and 360 have completed the tests, the BBC reported. The trial is scheduled to be completed in 2028.
Approximately 1 in 9 people over the age of 65 has Alzheimer's, including about 7 million people in the United States. It is the most common form of dementia, affecting memory, judgment skills and other cognitive functions. Early detection is important, as it offers access to medications that can slow decline and increases eligibility for clinical trials.
Doctors currently diagnose patients through cognitive exams, physical tests, interviews with loved ones, brain imaging and sometimes a spinal fluid test to check for amyloids and tau. The PET scans in particular can be expensive for patients, costing up to $3,000 without insurance in the United States.
"If this is successful, it provides a ubiquitous, accurate test which can detect the presence of abnormal amyloid protein in the brain without complicated, expensive investigations," Emer MacSweeney, a neuroradiologist who's recruiting U.K. volunteers, told the BBC.
In May 2025, the U.S. Food and Drug Administration approved a blood test from Malvern-based Fujirebio Diagnostics. It was the first of its kind to gain the federal clearance, however the blood is drawn through a syringe instead of a finger prick.
That test also measures the presence of amyloid and tau proteins, which can build up on the brain decades before Alzheimer's symptoms occur. It was about 92% as accurate in diagnosing patients compared to brain scans and spinal fluid tests. The FDA said that over 97% of those who tested negative for the blood test also tested negative for the other exams.