
November 18, 2020
 Mufid Majnun/via Unsplash
Mufid Majnun/via Unsplash
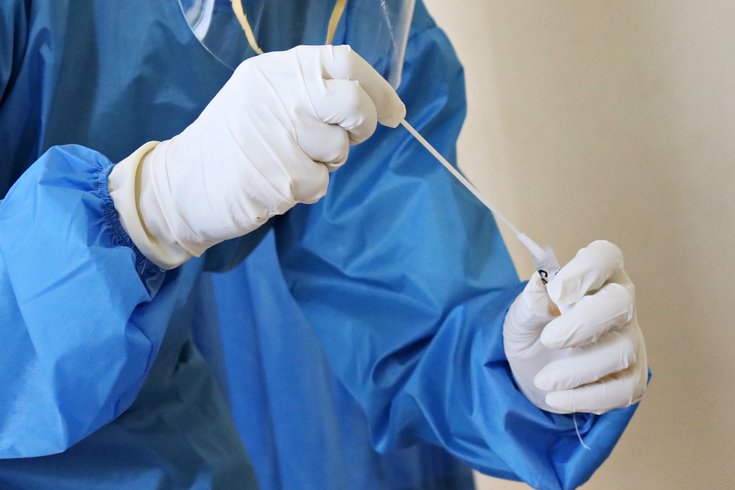
The at-home rapid COVID-19 test is conducted by swirling the self-collected nasal swab sample in a vial that is then placed in the test unit.
The U.S. Food and Drug Administration has issued an emergency use authorization for the first at-home rapid COVID-19 test, which can provide results within 30 minutes.
The all-in-one COVID-19 test kit, which was developed by Lucira Health, is a molecular single-use diagnostic that requires the self-collection of nasal swab samples, the FDA said in a statement.
The test is available to individuals age 14 and older who are suspected of having COVID-19 by their healthcare provider. They can self-administer the diagnostic from the comfort of their own home.
The diagnostic has also been approved for usage in point-of-care settings, which includes doctor’s offices, hospitals, urgent care centers, and emergency rooms. Anyone wishing to procure the at-home rapid COVID-19 test must have a prescription.
All ages are eligible to receive the test in a point-of-care setting, but samples must be collected by a healthcare provider when the diagnostic is being administered to children younger than 14 years old.
The test is conducted by swirling the self-collected nasal swab sample in a vial that is then placed in the test unit. The results can be read within 30 minutes from the unit’s light-up display, which shows whether an individual has tested positive or negative for COVID-19.
“The FDA continues to demonstrate its unprecedented speed in response to the pandemic. While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” said FDA Commissioner Dr. Stephen Hahn.
“This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission.”
Healthcare providers who prescribe the at-home rapid COVID-19 test are mandated to report all results to their respective public health authorities. Lucira Health also developed box labeling, quick reference instructions, and healthcare provider instructions to assist with reporting results.
“A test that can be fully administered entirely outside of a lab or healthcare setting has always been a major priority for the FDA to address the pandemic. Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” said Dr. Jeff Shuren, who serves as the director of the FDA’s Center for Devices and Radiological Health.
“We look forward to proactively working with test developers to support the availability of more.”
Follow Pat & PhillyVoice on Twitter: @Pat_Ralph | @thePhillyVoice
Like us on Facebook: PhillyVoice
Add Pat's RSS feed to your feed reader
Have a news tip? Let us know.